By: Rachel Levene, PhD Candidate in Molecular Microbiology at Tufts University School of Medicine and daughter of Dr. Eric Levene, Chester Pediatrics.
We have all heard a lot about the novel coronavirus or COVID19 testing lately. In fact, many of our public health experts and health professionals believe that having a strong and rapid testing infrastructure in place will be necessary before we can relax some of the social distancing protocols in place. This would allow us to quickly identify COVID19+ people in our community, isolate them and trace their contacts so that they can be isolated as well. This will help stop the spread and help us return a sense of normalcy. But where do we stand on our ability to test, treat, and even prevent COVID19?
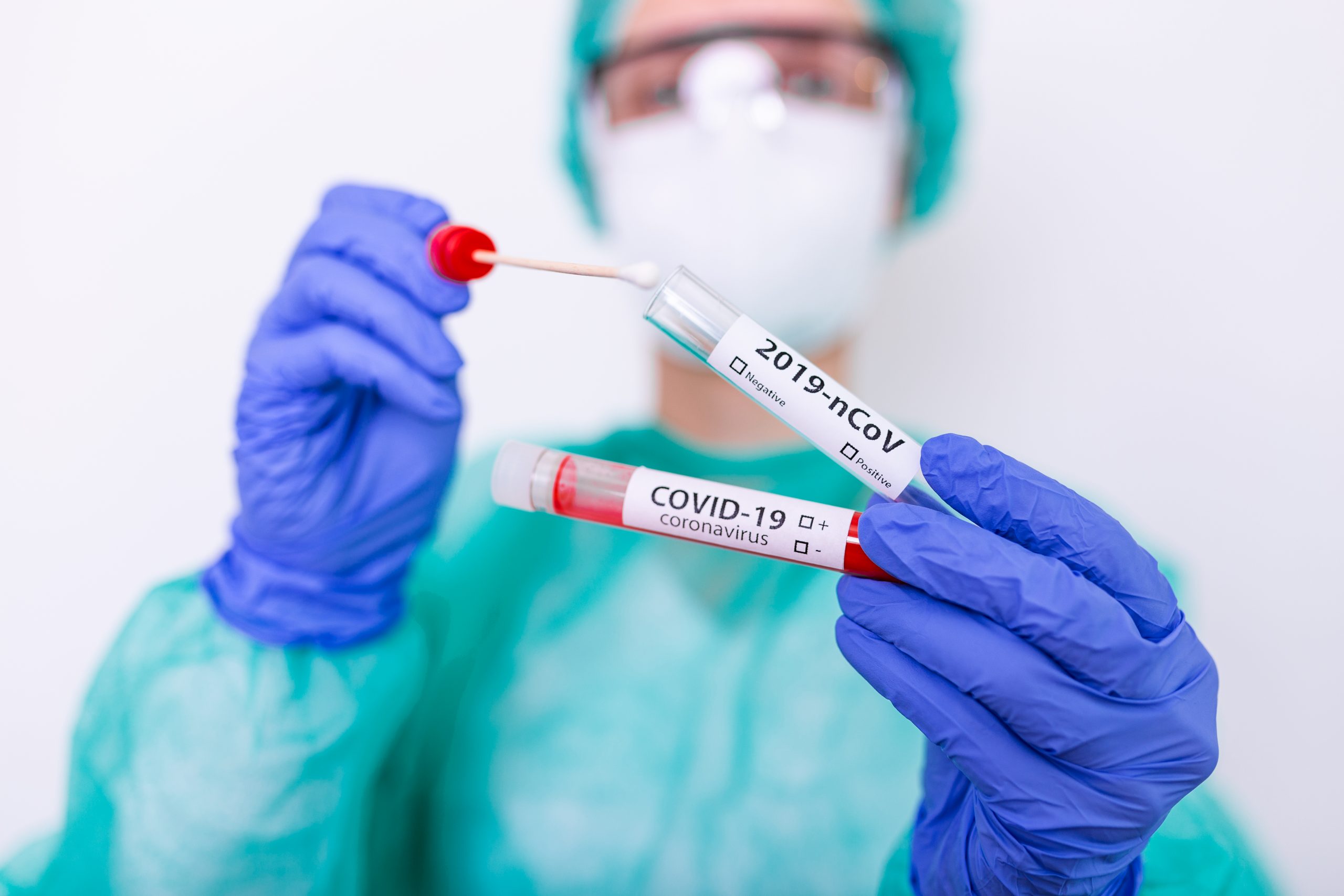
Molecular vs. Serology Testing: What’s the difference?
There are two main ways we have to test for COVID19: molecular and serology testing. The main difference between these two is that the molecular test looks for genetic material (RNA) from COVID19 and the serology test looks for our immune response to the virus in the form of antibodies. The molecular test can tell us if someone currently has the virus while the serology test just tells us if someone had the virus at some point and produced an immune response.
What is the molecular test?
The molecular test looks for the presence of the virus in a patient and is the main testing method used by the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), state and local public health labs, and commercial labs (like Quest Diagnostics). This is what is being done at drive-in test facilities. This test involves inserting a long swab into the back of the nose (nasopharyngeal) and then sending it off to the lab. The lab then performs a test called reverse-transcription polymerase chain reaction (RT-PCR) that detects pieces of the viral RNA. This test only takes a few hours, but results have been delayed because the labs are overwhelmed. While this is not ideal because it could take many days to get a positive result and start contact tracing, this test is the most accurate and is the least likely to give false negatives or false positives
Recently, the Food and Drug Administration (FDA) has given emergency use authorization (EUA) for molecular tests that are rapid and can give results in under an hour and will greatly expedite contact tracing and isolation. These are referred to as point of care testing and are very similar to the rapid strep or influenza test that is done in the doctor’s office. The most commonly discussed rapid molecular test is the one developed by Abbott Laboratories, but there are more that are being deployed across the country. They all involve the nasopharyngeal swab but instead of sending it off to the lab, it is inserted into a machine or cartridge that will analyze it and give a result quickly. While these tests are faster, they can occasionally give false positives or false negatives which are problematic for contact tracing and isolation.
What is the serology test?
When we get sick, our immune system makes highly specific antibodies to the pathogen that made us sick. The serology test looks for the presence of those antibodies to the coronavirus in a person’s blood. Unlike the molecular test, it can detect if someone had COVID19 and has recovered. It helps give us a picture of how COVID19 has spread in the community. This test is done via a blood draw that is sent off to a lab. The lab performs a test called ELISA that looks for antibodies specific to the coronavirus that causes COVID19. This is not point of care and could, therefore, take a couple of days especially if the lab is backlogged. There is an ongoing effort to develop a rapid point of care serology test that takes under an hour and can be done with a finger stick instead of a blood draw.
Why is there so much talk about serology tests? They identify individuals that had the virus and recovered or individuals that had an asymptomatic infection. The presence of antibodies suggests these people are immune to the virus, however it is unclear how long immunity lasts and if it is possible to get re-infected. Rapid deployment of serology testing could unveil that a lot more people than we thought had COVID19 and could, therefore, be immune. This could help inform decisions about relaxing social distancing.
Are there any treatments for COVID-19?
As of now, we have no approved drug that can directly target the virus. However, there are drugs and treatments in a clinical trial. A couple of the ones in active trials in the US are described below but there are more in clinical trials across the globe. Additionally, scientists are working tirelessly to develop new drugs and see if drugs that work on other viruses can be “repurposed” to treat COVID19.
Convalescent Plasma: Individuals who were sick with COVID19 made antibodies that helped them recover. These antibodies could also help individuals currently sick with COVID19 recover by boosting their ability to fight the virus. This was done for some Ebola patients treated in the United States and had some success. This treatment is currently in clinical trials across the nation including many in NYC. Another goal of this treatment is to determine if convalescent plasma can prevent exposed individuals from becoming sick.
This treatment requires individuals who recovered from COVID19 to donate plasma. If you had confirmed or presumptive COVID19 and it has been at least 14 days from your last day of symptoms contact the American Red Cross to donate.
Remdesivir: This medication is an antiviral drug that was developed to treat Ebola. It forces the machinery that copies the viral genome to prematurely stop replicating the viral genome which stops the virus from making more of itself. There are reports that patients who received Remdesivir through compassionate use recovered. Medications provided through compassionate use are given outside of clinical trial when then are no other treatment options. There are currently clinical trials being conducted to determine if Remdesivir is truly effective against COVID19.
Chloroquine and Hydroxychloroquine: These are drugs used to treat auto-immune diseases like lupus and rheumatoid arthritis. They work by dampening down the immune system, preventing it from attacking the patient’s own body. Why could these be effective against COVID19 if we need our immune system to fight the infection? The answer is that many patients with severe COVID19 have what is called a cytokine storm. The immune system goes into overdrive causing so much inflammation in the lungs that fluid fills the lungs causing respiratory failure. The hope is that chloroquine and hydroxychloroquine would dampen the immune response so it does not go into overdrive. However, this could also be detrimental because we need the immune system to fight the virus. Nonetheless, these medications are in a clinical trial as well.
When will we have a vaccine?
An approved vaccine is likely more than a year away. We first need to find a candidate vaccine that is safe and elicits a protective immune response. COVID19 is caused by a novel coronavirus and we have no existing template for coronavirus vaccines to build from. We do not know what is safe and effective. For example, in the case of the 2009 swine flu pandemic, we already knew how to make safe and effective influenza vaccines because we do it every year when we make each year’s vaccine. This allowed us to develop and produce a vaccine for large scale distribution so quickly. However, for COVID19 we are starting from “scratch” and need to figure out what will make a safe and effective vaccine. Scientists are working around the clock to get there. Once there is a promising vaccine candidate it needs to pass Phase I, II, and III clinical trials that fully evaluate safety and efficacy. After that, the infrastructure and manufacturing need to be ramped up for large scale production and distribution. As of now, there is a candidate vaccine that has begun a Phase I trial.
This is a difficult time, but we will get through it together. Reach out to your Allied Pediatrics pediatrician via telehealth for guidance and support. For children who are interested and want to learn more about viruses, vaccines, and our immune system encourage them to reach out to their science teachers.
To Donate Plasma:
Allied Physicians Group is a partnership of more than 150 dedicated, caring physicians and 350 highly trained support staff. Allied serves over 180,000 patients with offices throughout Greater New York City, Long Island, the Hudson Valley, and beyond. Founded in 2006, Allied Physicians Group is a recognized leader in increasing healthcare efficiencies and patient satisfaction, emphasizing support, innovation, and collaboration. If you are looking for a Pediatrician near you click here or for more information please visit https://alliedphysiciansgroup.com/.
